Welcome to the Theoretical Chemistry Group

News
- New paper out!
Congratulations, Eric, on your first paper in our group. Check out Eric's work on spin-polariton formation here.
https://doi.org/10.1063/5.0303448
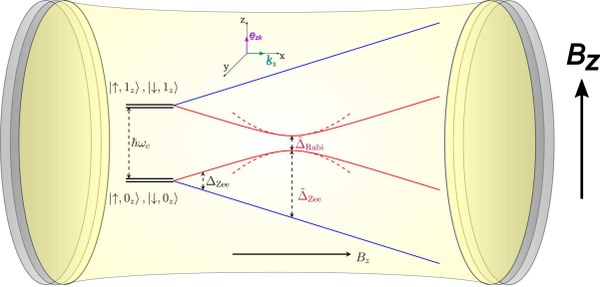
November 2025
- Hiking Adventure in the woods around Berlin's second largest mountain, Müggelberg, and along the bank of the river Dahme.

October 2025
- New paper out!
Congratulations, Philipp, on your paper about the structure and reactivity of a heterobimetallic Ir/Pd complex.
https://pubs.rsc.org/doi.org/10.1039/D5SC03892H
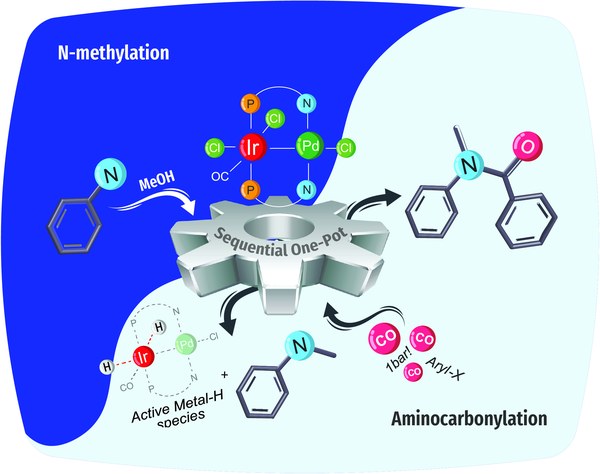
September 2025
- Our team hosted the 7th Quantum Bio-Inorganic Quantum Chemistry Conference (QBIC VII). We experienced lots of great talks and intense discussions. QBIC society awards went to Anastassia Alexandrova (UCLA) and Mariusz Radon (Cracow). Thanks to the whole team and our sponsors: Faccts, Turbomole, NOMADLab and ACS.

August 2025
- "Forschung und Lehre" has published a report about "DigiMatCh", our project about digital education in math for chemists. The report includes parts of an interview with Michael and Prof. Vera Krewald from TU Darmstadt.
https://www.forschung-und-lehre.de/lehre/wie-individuell-kann-lehre-sein-7211
August 2025
- New paper out!
Congratulations, Leon, on publishing the first of your Co-papers.
https://onlinelibrary.wiley.com/doi/10.1002/anie.202503705
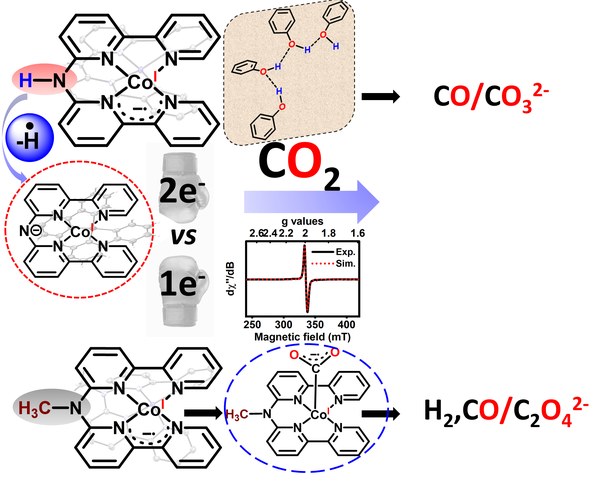
May 2025
- DigiMatCh project receives funding from „Stiftung Innovation in der Hochschullehre“
We are happy to announce that our project „Digitallabor Mathematik für die Chemie“ will be supported within the „Freiraum 2025“ funding of the „Stiftung Innovation in der Hochschullehre“. The aim of the project is to develop digital computer experiments that enable chemistry students an easier access to mathematical knowledge and visualization skills in chemistry.

April 2025
- This year's HUMMR hackathon was going down in Burg/Spreewald. It was remarkably productive while we could still enjoy ourselves in a very nice location.

March 2025
- New Paper out!
Congratulations, Mihkel, the long-awaited nuclear gradients paper is out.
https://pubs.acs.org/doi/10.1021/acs.jctc.5c00021
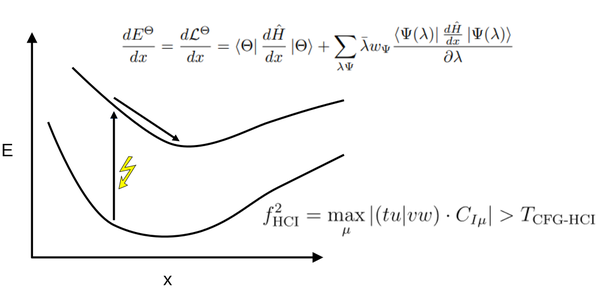
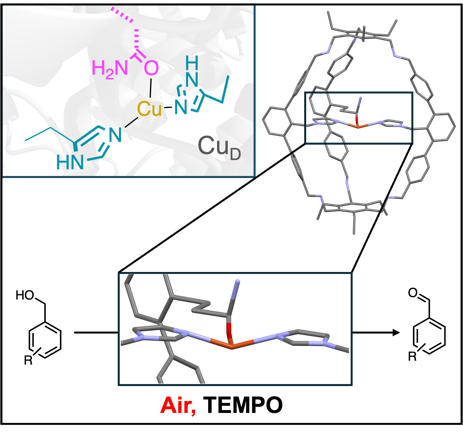
- New Paper out!
Congratulations, Leon, on your paper about local structure of a biomimetic Cu cage complex.
https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/chem.202500533
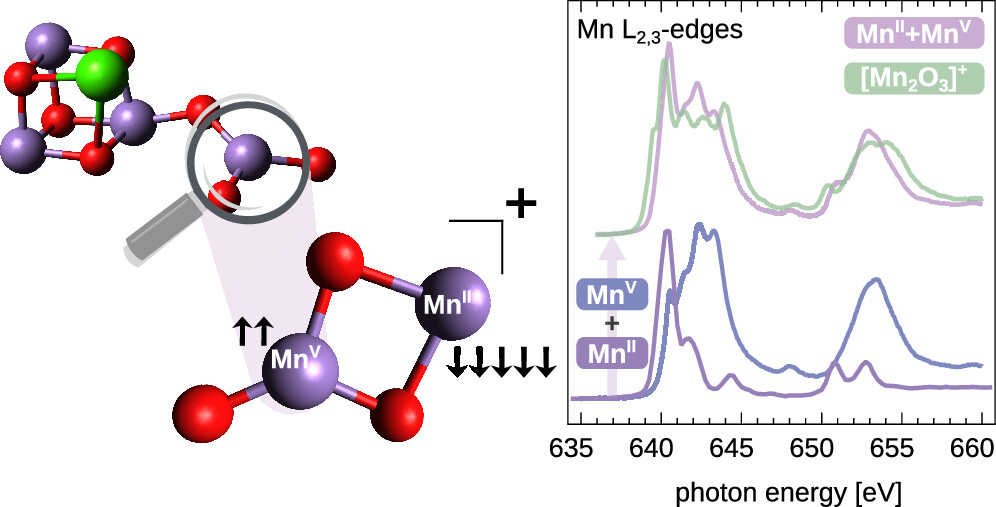
New paper out!
Congratulations, Mihkel and Esma, on your paper about a the electronic structure of a strongly correlated Mn dimer. https://pubs.acs.org/doi/10.1021/jacs.4c14543
***Press release***
Congratulations Dr. Ugandi!
Congratulations, Mihkel, on an excellent dissertation and defense on the "Implementation of multiconfigurational quantum chemistry methods".

July 2024
-
Welcome back Lucas!
After his guest visit in 2022, Dr. Lucas Welington de Lima rejoins the group as Postdoc, investigating the interactions and reactions between water and mixed metal oxide clusters in the gas phase. This study will be conducted in collaboration with the research teams led by Prof. Dr. Dr. h.c. Joachim Sauer and Prof. Dr. Knut R. Asmis.
October 2024
-
Dissertation defended!
Congratulations, Esma on an excellent defense. We wish you all the best for your future @NOMAD Lab, Dr. Boydas.

July 2024
-
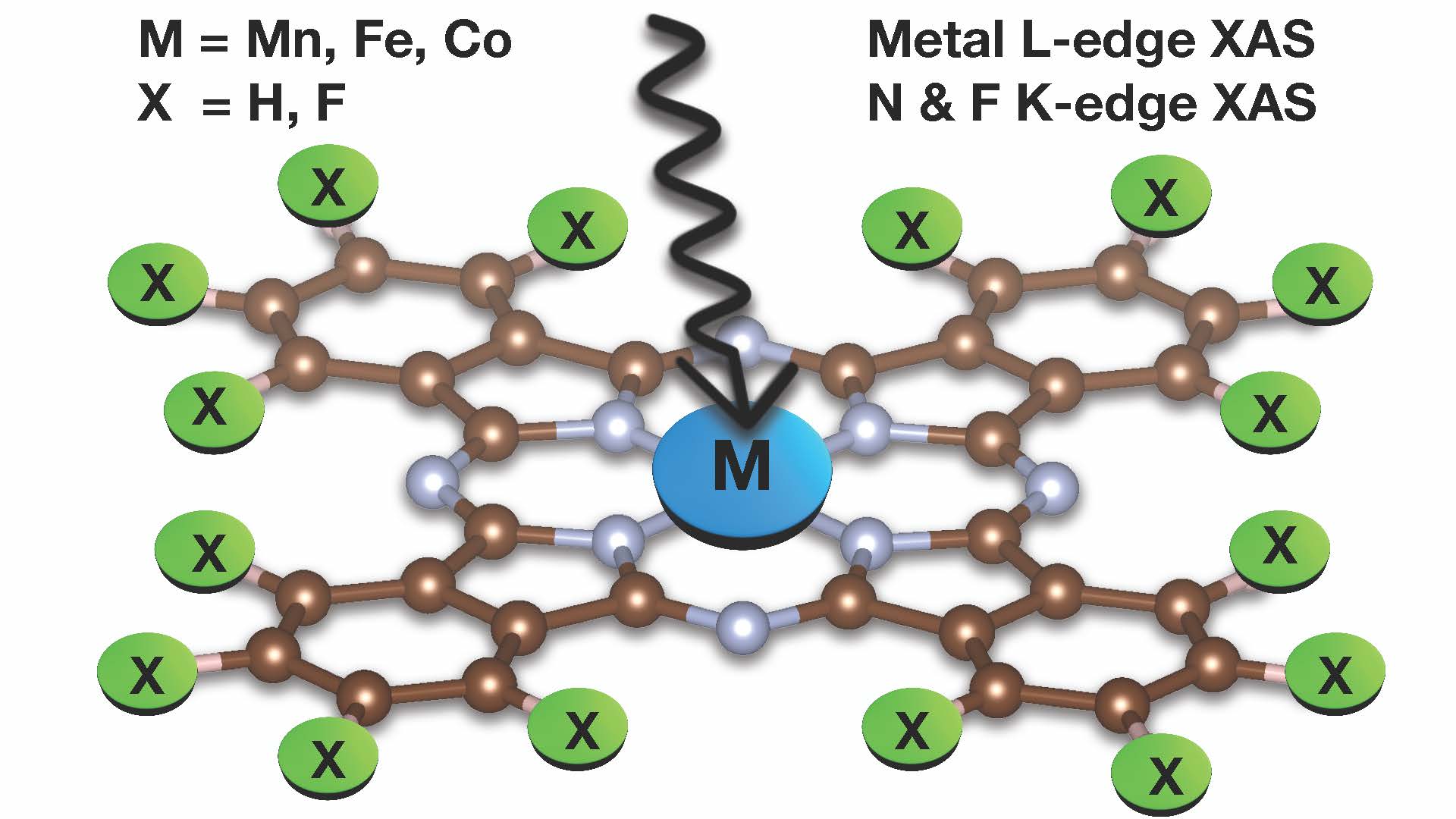
New Paper out!
Congratulations, Esma, on your paper about modelling X-Ray absorption spectra of transition metal phtalocyanines. https://pubs.rsc.org/doi.org/10.1039/D4CP01900H
July 2024
-
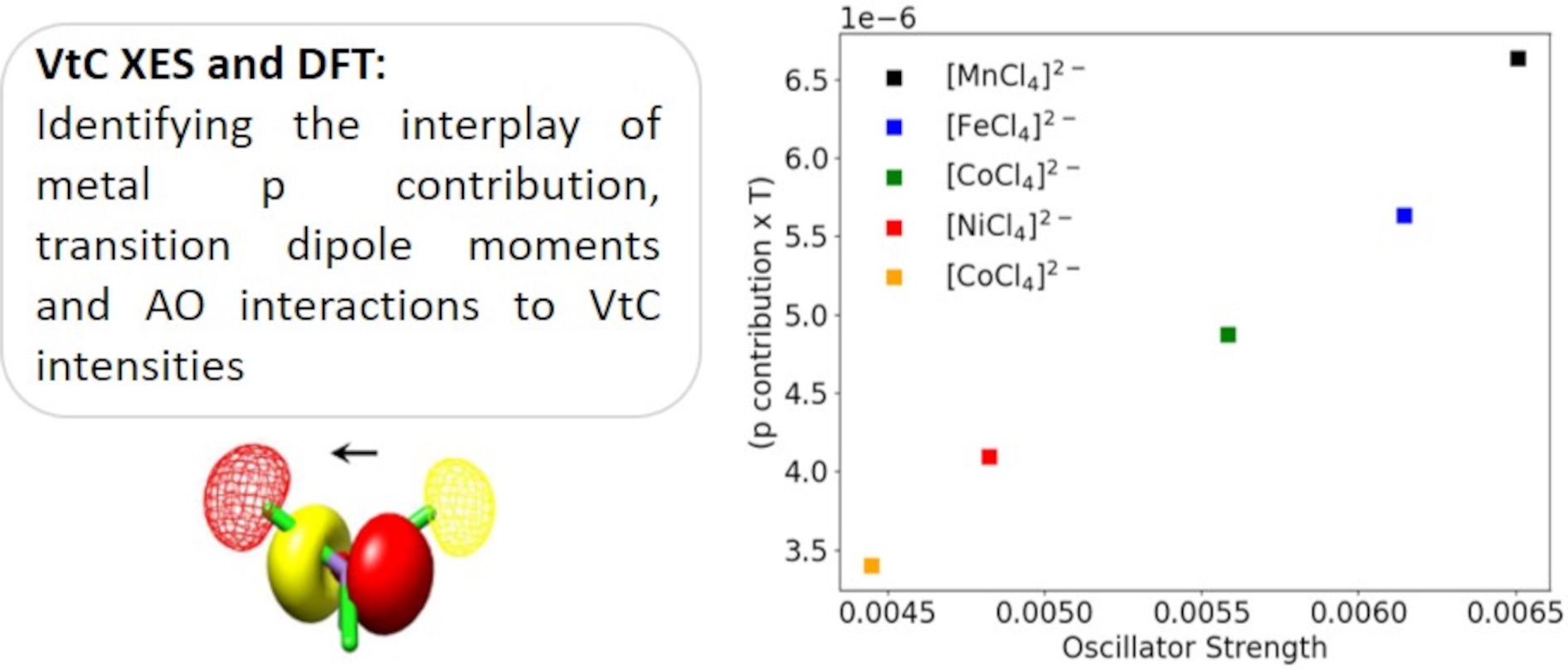
New Paper out
Finally, the second article by Tina and Michael Roemelt was accepted. https://pubs.rsc.org/doi.org/10.1039/D4CP00967C
June 2024
-
New Paper out!
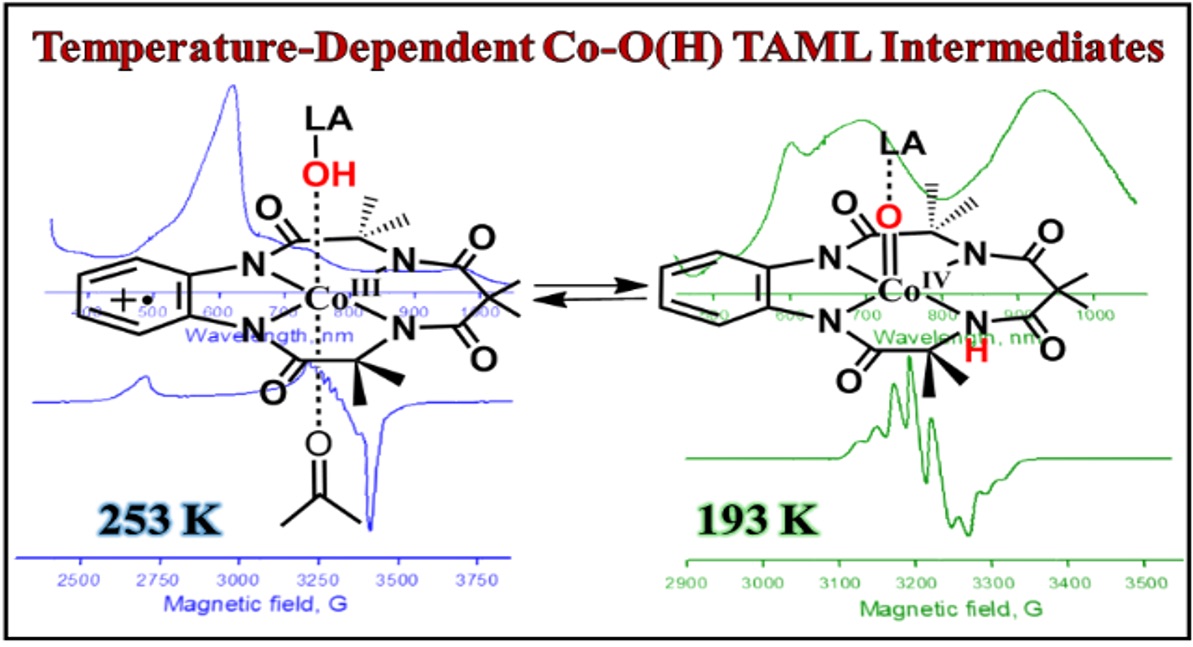
Congratulations, Gurjot, on your paper about the electronic and geometric structure of two interconvertible Cobalt–Oxygen TAML compounds. https://pubs.acs.org/doi/10.1021/jacs.3c14346
May 2024
-
Bachelor Thesis defended!
We congratulate Eugenio to the very succesful defence of his bachelor's thesis.
April 2024
-
HUMMR-Hackathon 2024
The first of hopefully many HUMMR-hackathons in Warnemünde was a blast.

March 2024
-
New Paper out!
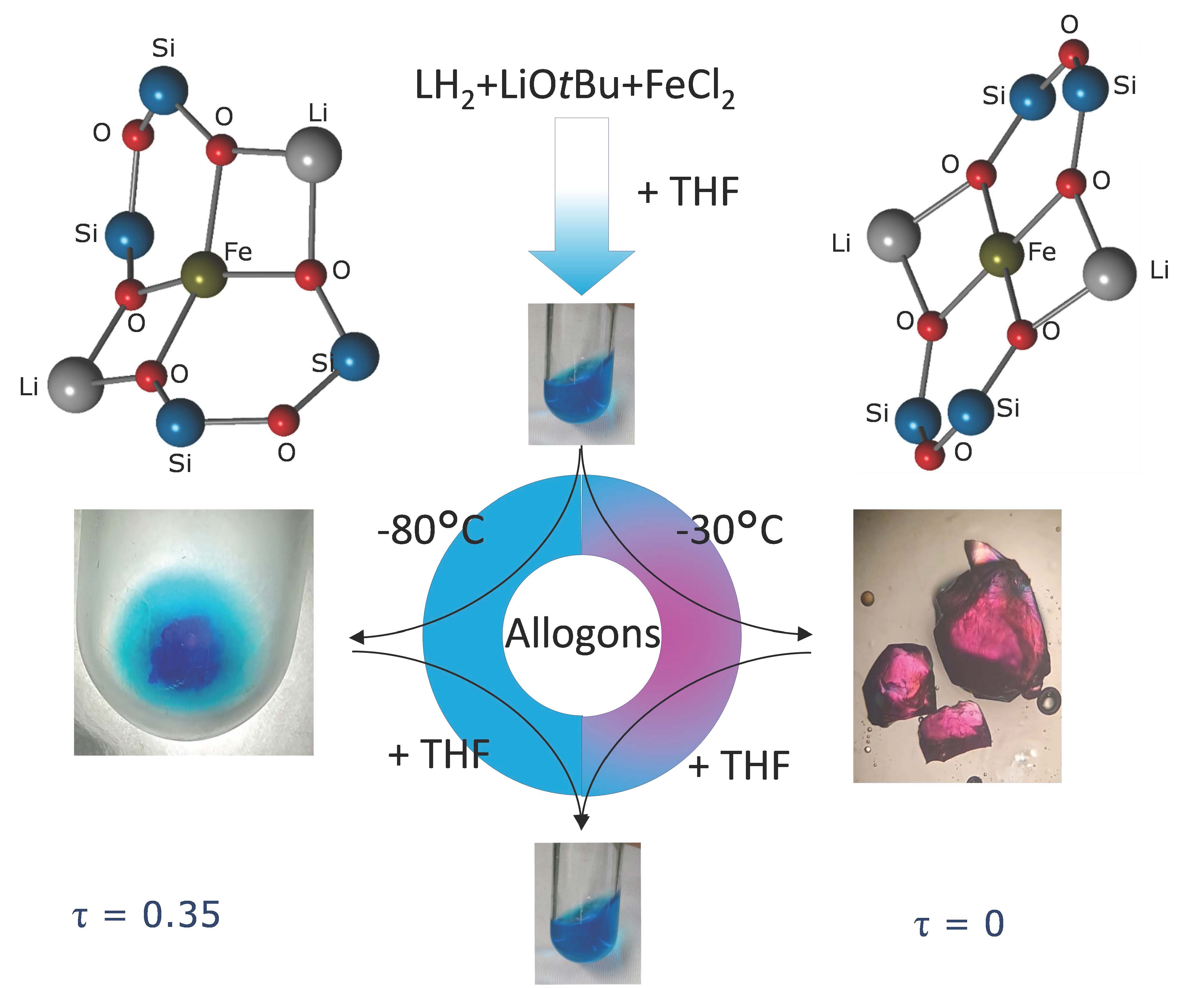
Congratulations, Philipp, on your paper about selectively crystallized Bis(disiloxido)ferrate(II) allogons and their properties https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/chem.202303614
December 2023
-
Congratulations, Eric, on being accepted for the Walter Benjamin Programme of the DFG
The research project aims at a microscopic understanding of thermal reaction mechanisms and molecular spin properties in polaritonic chemistry. Ab initio electronic structure methods will be developed for closed- and open-shell molecules strongly coupled to low-frequency optical cavities.

December 2023
-
New Paper out!
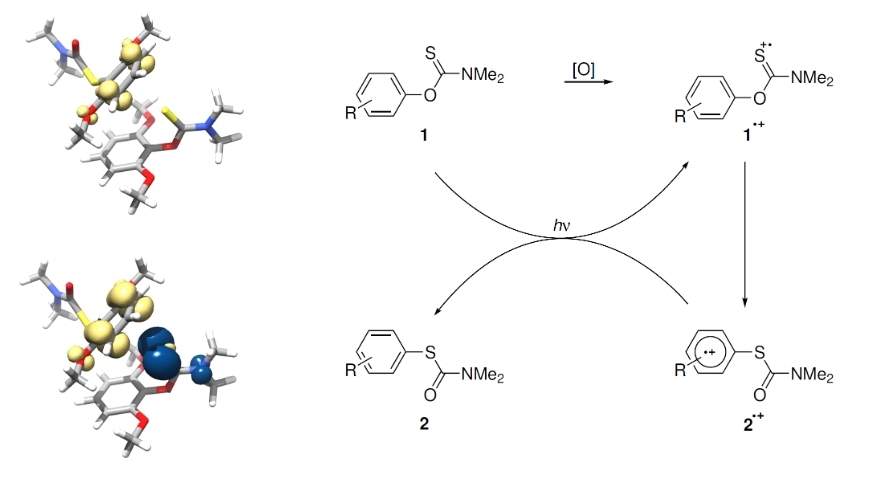
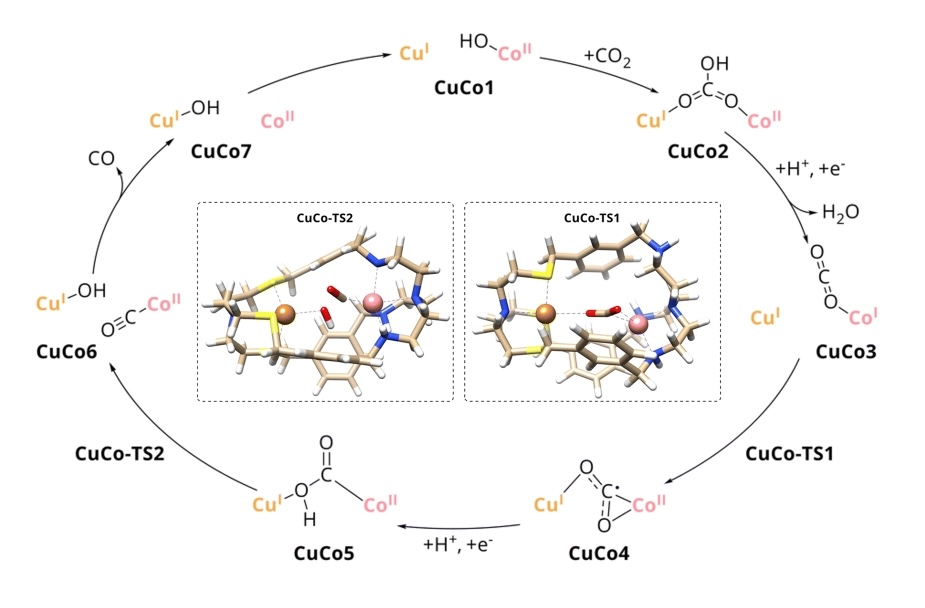
Congratulations, Esma, on your paper about light-driven reduction of CO2
https://pubs.rsc.org/en/content/articlehtml/2023/sc/d3sc02679e
November 2023
-
Welcome to the group, Dr. Eric Fischer.
Dr. Eric Fischer joins the Quantum Chemistry Group as a guest scientist for four month.
September 2023
-
Presentation Award at the QBIC VI
Congratulations to Esma on winning the prize for the best oral presentation at the 6th Quantum Bio-Inorganic Chemistry Conference in Warsaw.

September 2023
-
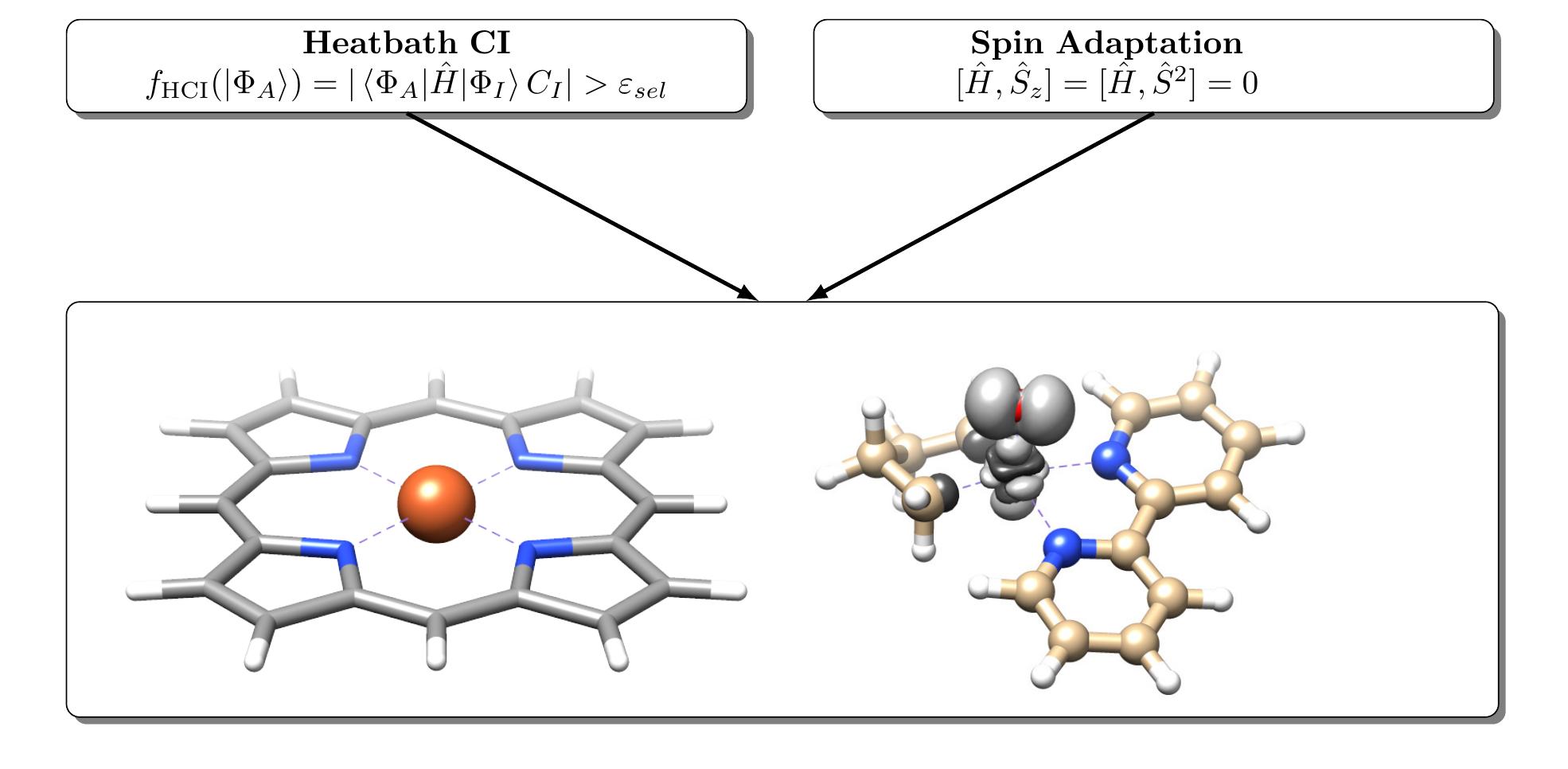
Congratulations, Mihkel, on your paper about spin-adapted heatbath-CI, August 2023.
https://onlinelibrary.wiley.com/doi/full/10.1002/jjc.27203
September 2023
-
Good news: Our proposal for a new computer cluster has been accepted by the DFG
August 2023
