Exploring the Limits of π-Aromaticity
|
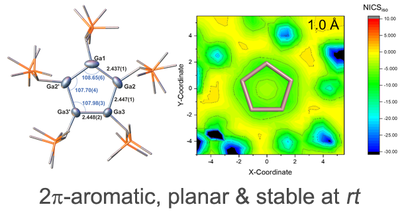
Most often, aromatic rings contain six or more π-electrons, such as in benzene or the cyclopentadienyl anion. In contrast, examples of 2π-aromatics are very rare and remain limited to three- and four-membered rings. Now the groups of Robert Kretschmer (Chemnitz) and Oliver Dumele (HUB) report on a cyclopentagallene dianion (pictured), which features a unique example of a five-membered aromatic ring stabilized by only two π-electrons. The team quantified the ring current computationally and the results substantiate the proposed aromatic character of the Ga5 ring and suggest that aromatic stabilization can occur in species that had previously been considered below the minimum π-electron count in a five-atom ring fragment. Link to publication: A Planar Five‐Membered Aromatic Ring Stabilized by Only Two π‐Electrons, Angew. Chem. Int. Ed. 2022, https://doi.org/10.1002/anie.202206963 |