Platinum-Catalysed Hydrofluorination of Alkynes at Room Temperature Promoted by a Fluoride Shuttle
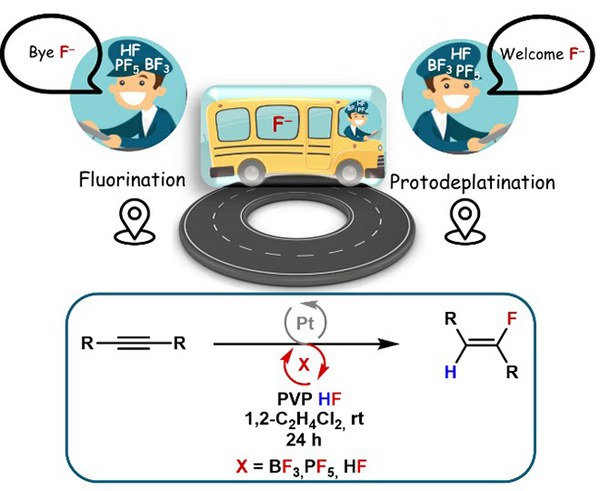
The Braun group reported on a platinum-catalysed hydrofluorination system that gives selectively (Z)-fluoroalkenes. The mechanism was elucidated using a combined experimental and computational approach. The catalysis is characterised by an interplay between the a cationic Pt(II) species and an interaction of the fluorinated anion with HF. It has been shown that BF3, PF5 and HF can shuttle a fluoride anion to a metal-coordinated alkyne, enabling the catalytic hydrofluorination of alkynes. The resulting fluoroalkene is considered a bioisoster of amide functional groups. As a proof of concept, a bioisosteric molecule of paracetamol was synthesised successfully. This new methodology opens up possibilities for the synthesis of a variety of novel fluoroalkenes and offers potential for applications in areas such as pharmaceuticals and materials science.
Link to the original publication:

