Limberg / Kaupp Group
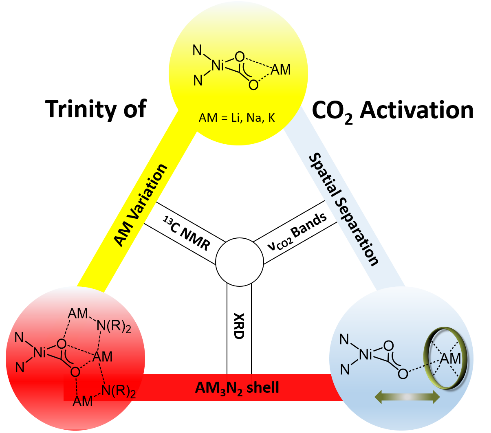
The Limberg group has shown in cooperation with the Kaupp group (TUB) that in bioinspired nickel-CO2 complexes the degree of CO2 activation depends on the nature of the alkali metal counterion, the strength of its interaction with the CO2 unit – and thus its special separation – as well as on further polar entities in the second coordination sphere. This is also reflected in the reactivity towards electrophiles which lead to C-O bond cleavage and CO formation.
S. Wolff, V. Pelmenschikov, R. Müller, M. Ertegi, B. Cula, M. Kaupp, C. Limberg, Chem. Eur. J. 2024. e202303112; "Controlling the Activation at NiII-CO22- Moieties through Lewis Acid Interactions in the Second Coordination Sphere"
Link to the original publication: https://chemistry-europe.onlinelibrary.wiley.com/doi/epdf/10.1002/chem.202303112
The paper is part of special collections on Modern Lewis Acid Chemistry and Hot Topic: Carbon Dioxide