Synthesis of Hydroperoxides
Geminal Dihydroperoxides are organic compounds containing two hydroperoxy-moieties at the same carbon atom. While the first compounds of this class have been synthesized more than 50 years ago, only in the last 10-15 years were systematic efforts taken to find routes of synthesis that are applicable to make a broad spectrum of gem-dihydroperoxides. This renewed interest is mostly due to the new-found antimalarial activity of 1,2,4,5-tetroxanes, a substance class that is usually synthesized starting from gem-dihydroperoxides.
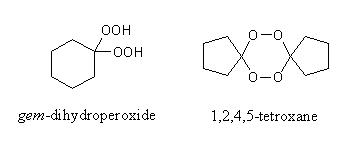 |
The goal of the research in this group is to find ways to easily synthesize gem-dihydroperoxides and/or related substance classes, learn more about the reactive behaviour of them as well as use hydroperoxides, gem-dihydroperoxides or their derivatives in oxygen-transfer reactions, such as epoxidation or the preparation of sulfoxides. If possible, new reactions should be established to even stereoselectively conduct these oxygen transfers.
Example of recent works:
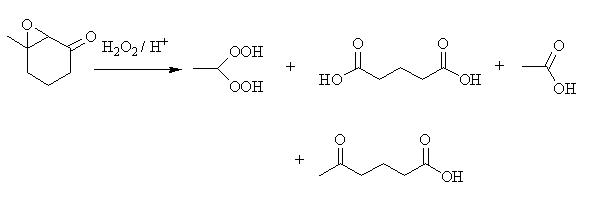
Substituted 2,3-Epoxyketones could be shown to fragment, under the acid-catalyzed influence of hydrogen peroxide, into various products, for instance mono-and dicarboxylic acids, ketoacids, and also gem-dihydroperoxides. Among the gem-dihydroperoxides thus synthesized was also ethane-1,1-dihydroperoxide, so far the compound with the highest amount of peroxidic oxygen described in the literature.
 |
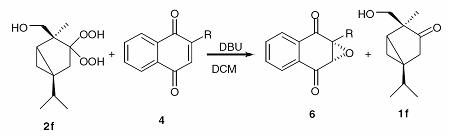
gem-Dihydroperoxides can be used to epoxidize different substrates. New DHPs, derived mostly from terpenes, were synthesized. With these, substituted 1,4-naphthoquinones were stereoselectively oxidized to the corrseponding epoxynaphthoquinones with yields up to 97% and ee's up to 82%.
 |
Selected Publications
Alexander Bunge, Hans-Jürgen Hamann, Dennis Dietz, Jürgen Liebscher: "Enantioselective epoxidation of tertiary allylic alcohols by chiral dihydroperoxides"
Tetrahedron, 2013, 69, 2446-2450
Hans-Jürgen Hamann, Mandy Hecht, Alexander Bunge, Malgorzata Gogol, Jürgen Liebscher: "Synthesis and antimilarial activity of new 1,2,4,5-tetroxanes and novel alkoxysubstituted 1,2,4,5-tetroxanes derived from primary gem-dihydroperoxides"
Tetrahedron Lett., 2011, 52, 107-111
Alexander Bunge, Hans-Jürgen Hamann, Jürgen Liebscher: "A simple, Efficient and Versatile Synthesis of Primary gem-Dihydroperoxides from Aldehydes and Hydrogen Peroxide"
Tetrahedron Lett., 2009, 50, 524-526
Alexander Bunge, Hans-Jürgen Hamann, Eve McCalmont, Jürgen Liebscher: "Enantioselective epoxidation of 2-substituted 1,4-naphthoquinones using gem-dihydroperoxides"
Tetrahedron Lett., 2009, 50, 4629-4632
Hans-Jürgen Hamann, Alexander Bunge, Jürgen Liebscher: "Reaction of Epoxyketones with Hydrogen Peroxide - Ethane-1,1-dihydroperoxid as a Surprisingly Stable Product"
Chemistry Eur. J., 2008, 14, 6849-6851
H.-J.-Hamann, M. Chmielewski, D. Mostowicz, J. Liebscher "Enatioselctive epoxidation of 1-hexene with sugar derived hydroperoxides"
Arkivoc, 2007, ix, 17-20
Hans-Jürgen Hamann, André Wlosnewski, Tonino Greco, Jürgen Liebscher: "Novel Hydroperoxydioxolanes and -dioxanes by Hydroperoxide-Rearrangement and Ozonolysis"
Eur. J. Org. Chem., 2006, 2174-2180
H.-J. Hamann, J. Liebscher: "A Novel Outcome of the Hydroperoxide-Rearrangement"
J. Org. Chem., 2000, 65, 1873-1876
