Spectroscopic Trapping of Reactive Intermediates by Small Molecule Activation
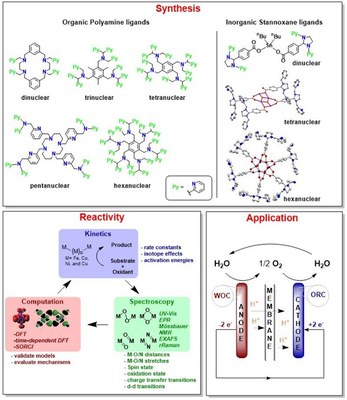
Nature often employs polynuclear metal active sites involving cheap and abundant metals like Mn, Fe, and Cu to achieve vital functions like water oxidation, ammonia synthesis, or dioxygen reduction. Artificial catalysts that are similarly efficient and based on abundant materials are of great interest. However, only a relatively small number of molecular catalysts are hitherto known, with the majority of them being based on expensive second and third row transition metals. Moreover, in most cases the known compound is a precatalyst and the precise species responsible for catalytic activity is not known. To engineer further improved catalysts, there is a substantial impetus to characterize fully the active species of existing complexes, including all relevant higher oxidation states, intermediates, and transition states, so as to establish the mechanism(s) by which they affect activation of small molecules. In our group we aim to investigate systematically the mechanism of the activation of small molecules at transition metal centers, which is a common strategy applied in biology for achieving key metabolic functions. Our group is specialized in organic and organometallic synthesis, spectroscopic trapping of reactive intermediates by Mössbauer, X-ray absorption, resonance Raman, electron paramagnetic resonance, and temperature dependant UV-Vis absorption spectroscopic methods. Extremely transient intermediates are trapped by cryo‐stopped‐flow experiment using a diode‐array UV/Vis spectrometer. By employing a combined synthesis, spectroscopy and computational (in collaboration with other groups) approach our group aims at obtaining vital insights into the prerequisites necessary for the design of efficient catalysts for the selective functionalization of unactivated C–H bonds, O2 reduction and water oxidation by using cheap and readily available first-row transition metals under ambient conditions. The long term aim of our group is the design of cost-efficient fuel cells based on renewable energy sources. The bioinorganic systems we study can be divided into three general areas:
Angew. Chem. Int. Ed. 2011 Angew. Chem. Int. Ed. 2014 Nature Communication 2017
Chem. Comm. 2012

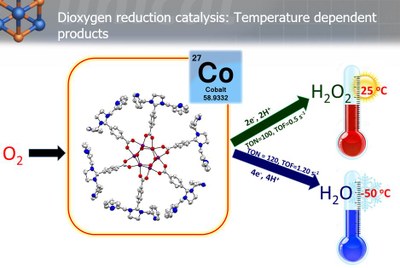
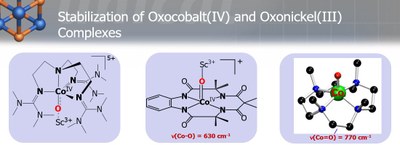
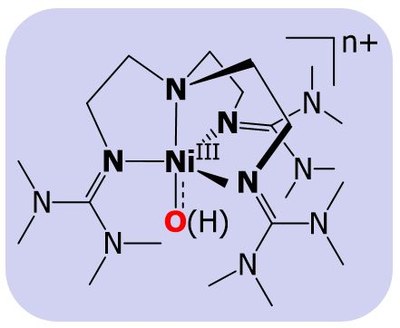
Nature has used metalloenzymes for the selective functionalization of strong C−H bonds as well as the oxidation of water in atom‐economical transformations that employ cheap and nontoxic metals and operate under ambient pressure and temperature. High‐valent metal–oxo cores have been proposed, and in a few cases isolated, as the common reactive intermediates in these biological reactions relevant to the formation of renewable energy. Although the chemistry of high-valent metal-oxo species has been well advanced in early transition metals there has been a long-standing, challenging task for the synthesis and characterization of high-valent metal-oxo complexes of the late transition metals, such as Co, Ni, and Cu. Nevertheless, they are attractive synthetic targets, as they are considered as potential reactive intermediates in various hydrogen atom abstraction (HAA) and in group transfer (GT) reactions with unsaturated substrates. In the last few years our group has employed some novel strategies to stabilize such elusive intermediates, responsible for catalytic oxidation reactions.
Angew. Chem. Int. Ed. 2017
Angew. Chem. Int. Ed. 2013

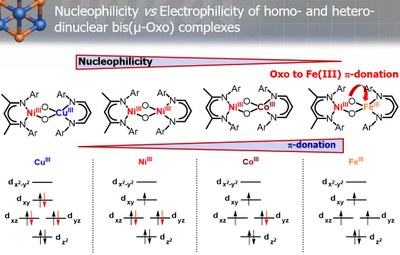
Homo- and heterodinuclear Bis(µ-oxo) complexes of iron, nickel, cobalt and copper have been proposed as reactive intermediates in various catalytic electrophilic and nucleophilic oxidation reactions. However, the factors that control the electrophilic vs nucleophilc reactivity of these cores were not understood properly. In our group we synthesized a series of heterodinuclear bis(µ-oxo)NiIIIMIII complexes and characterized them by a variety of spectroscopic methods. Furthermore, complementary theoretical and reactivity studies have helped to establish a structure-function correlation for the reactivity of these biologically relevant intermediates.

Nam; Que et. al. Science 2003 Angew. Chem. Int. Ed. 2017

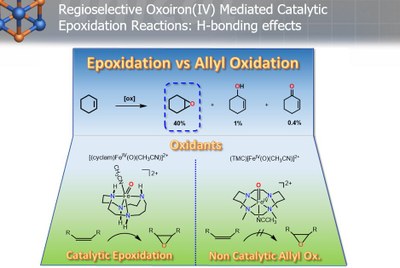
A functional model of the biological high-valent iron(IV)oxo species has been synthesized and spectroscopically characterized in our group. The ligand employed is a modified cyclam ligand, where one of the nitrogens in replaced by oxygen.
Angew. Chem. Int. Ed. 2016 J. Am. Chem. Soc. 2012
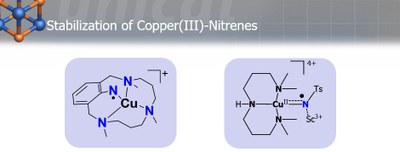
The development of new and more effective catalysts invariably hinges on an improved understanding of the intermediates formed by the catalyst as it works on the reagents. Chemists had previously suggested that certain copper-catalyzed reactions might involve high-valent, terminal copper–nitrene units in aziridination and amination reactions but these species had remained elusive for decades ensuring that the mechanism of such reactions remained hidden. In our group (also in collaboration with the group of Dr. Anna Company, University of Girona) we used novel methods to trap two such copper-nitrene intermediates. The research suggests that Cu(III)NTs – an unusual oxidation state for the metal two levels above copper(I) – are the core component of the copper-based oxidation catalysis used in aziridination and amination reactions. The work may also provide insights into the catalytic cycle of mononuclear copper monooxygenases.

J. Am. Chem. Soc. 2014
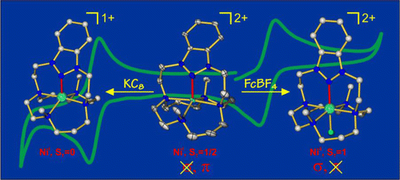
In this study we have shown the N-heterocyclic nitrenium ligand, which is isoelectronic to the well-known N-heterocyclic ligands, as a potentially useful and versatile reagent in transition-metal-based catalysis.
Spectroscopy/Techniques
Electron Paramagnetic Resonance (EPR)
X-Ray Absorption (XAS)
Stopped Flow
Electro-Chemistry
Gas Chromatography - Mass Spectrometry (GC-MS)
Mass - Advion
Mössbauer (in cooperation with Prof. Limberg's Group)
Glovebox and Schlenk Line

|