Research
What are we dealing with?
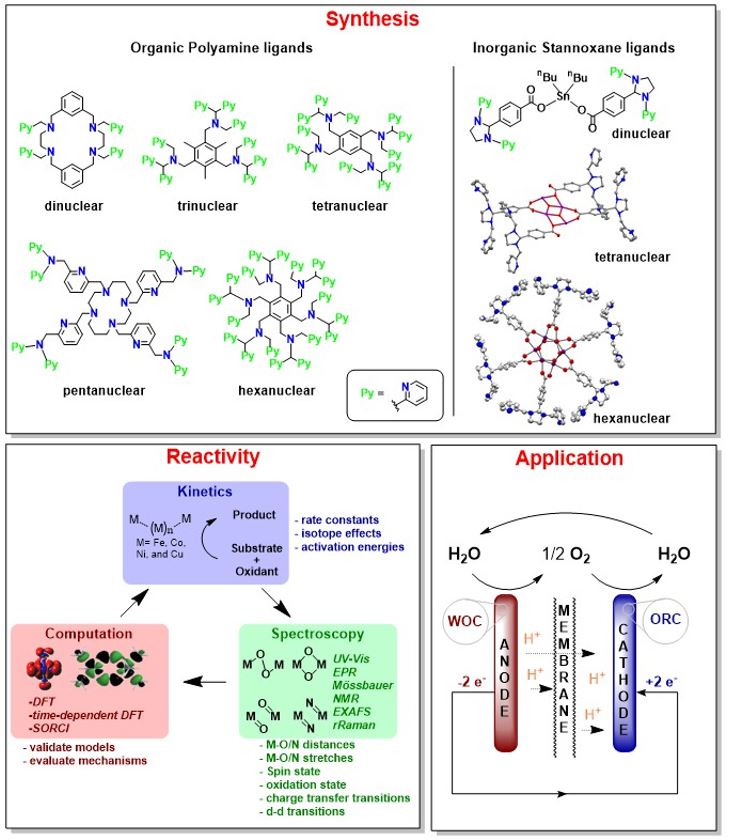
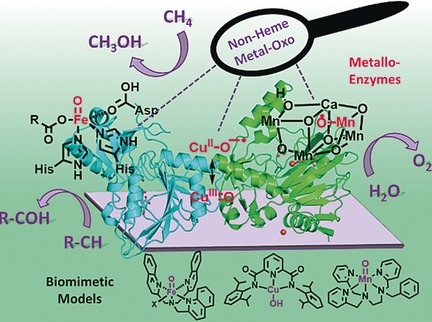
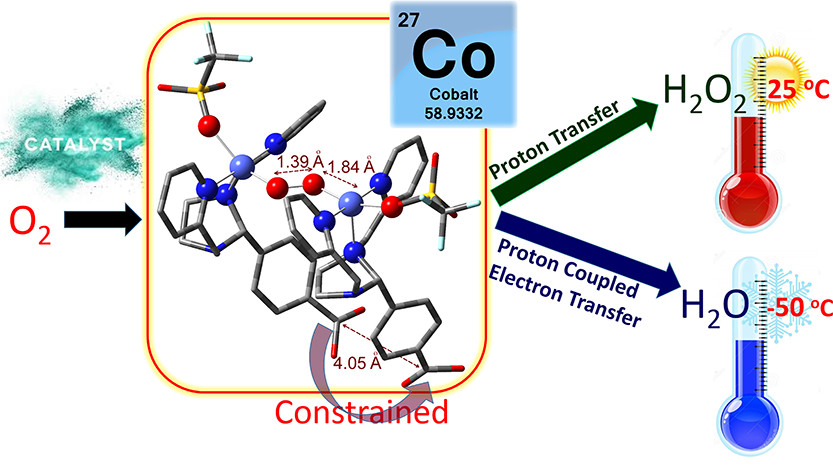
Our research targets the enlightenment of mechanistic details of the activation of small molecules (O2, H2O, CO2) for redox processes at enzyme like catalysts with transition metal centers e.g. Fe, Cu, Co, Mn and Ni.
What is our motivation?
The nature uses various enzymes for oxidative and reductive metabolism processes in a very efficient way. The present metal ions are neither rare nor expensive. On the other side, for artificial industrial reactions one needs catalysts with active centers based on rare and noble metals. Thats why synthetic catalysts which react in a similiar efficient way like the natural enzymes but are based on viable metal inlike the present industrial catalysts are in hugh interest. Furthermore, mostly the catalytically active species - an intermediate - is not known.
Thats what our research is into!
How do we do it?
Our synthesized transition metal complexes will be activated by small molecules e. g. O2, CO2 or H2O. Due to the decrease of the reaction temperature the reaction can be monitored by UV/Vis spectroscopy. Therefore, reactive intermediates can be trapped and analysed with further analytical techniques.
For that we use a variety of different anayltical techniques:
Other analytical methods
- Mößbauer spectroskopy (Maximilian Schütze, Hivda Özgen)
- Resonance Raman spectroscopy (Maximilian Schütze, Hivda Özgen, Clemens Schleeh)
- X-ray absorption spectroscopy (Maximilian Schütze)
- single crystal X-ray diffraction analysis (Dibya Jyoti Barman)
What do we achieve?
We can obtain the oxidation and spin states through which the metal ion is going and what specifically matters for the reactivity. The combination of synthesis, spectroscopy and quantum mechanical calculations - also in collaboration with various other groups around the world - gives important insights into the reaction mechanisms. The knowledge about mechanistical details is a requirement for the design of efficient catalysts for targeted reactions e.g. oxygen reduction, water oxidation, carbondioxide activation or water splitting for genereation of molecular hydrogen.

![epr picture.jpg [alternativer Bildtext]](https://www.chemie.hu-berlin.de/de/forschung/ray/alt/copy_of_forschung/epr-picture.jpg/@@images/image-360-bc02787c53700265f3ae4cfff8915117.jpeg)
![glove box picture.JPG [alternativer Bildtext]](https://www.chemie.hu-berlin.de/de/forschung/ray/alt/copy_of_forschung/glove-box-picture.jpg/@@images/image-360-bc02787c53700265f3ae4cfff8915117.jpeg)
![ESI-MS Advion.JPG [alternativer Bildtext]](https://www.chemie.hu-berlin.de/de/forschung/ray/alt/copy_of_forschung/esi-ms-advion.jpg/@@images/image-360-bc02787c53700265f3ae4cfff8915117.jpeg)
![GC-MS.JPG [alternativer Bildtext]](https://www.chemie.hu-berlin.de/de/forschung/ray/alt/copy_of_forschung/gc-ms.jpg/@@images/image-360-bc02787c53700265f3ae4cfff8915117.jpeg)
![UVVis.JPG [alternativer Bildtext]](https://www.chemie.hu-berlin.de/de/forschung/ray/alt/copy_of_forschung/uvvis.jpg/@@images/image-360-bc02787c53700265f3ae4cfff8915117.jpeg)
![Electrochem [alternativer Bildtext]](https://www.chemie.hu-berlin.de/de/forschung/ray/alt/copy_of_forschung/electrochem/@@images/image-360-a839fb66f8a31a5c9c6bea9408c8d631.jpeg)
![FTIR 3.png [alternativer Bildtext]](https://www.chemie.hu-berlin.de/de/forschung/ray/alt/copy_of_forschung/ftir-3.png/@@images/image-360-bc02787c53700265f3ae4cfff8915117.png)
![Stopped Flow.JPG [alternativer Bildtext]](https://www.chemie.hu-berlin.de/de/forschung/ray/alt/copy_of_forschung/stopped-flow.jpg/@@images/image-360-bc02787c53700265f3ae4cfff8915117.jpeg)